Ever been fascinated by those sparkling blue crystals in your chemistry lab? Those are copper sulphate crystals—known for their brilliant blue color, geometric shape, and significance in educational chemistry experiments.
In this blog, we’ll describe how crystals of copper sulphate are prepared at home or in a laboratory, along with safety measures, materials needed, and the science behind it.
Whether you’re a student preparing for your chemistry practical or a teacher looking for an educational demonstration, this guide will walk you through everything you need to know.
What is Copper Sulphate?
Copper sulphate is a blue, crystalline salt with the chemical formula CuSO₄·5H₂O (copper (II) sulphate pentahydrate). It is widely used in agriculture, chemistry labs, electroplating, and even in school science projects.
Key Properties:
- Color: Bright blue
- Solubility: Soluble in water
- Appearance: Crystalline solid
Why Learn to Prepare Copper Sulphate Crystals?
Learning to prepare copper sulphate crystals teaches you:
- The basics of crystallization, a method of purification
- Scientific handling of chemicals
- How temperature and saturation affect crystal growth
This experiment is ideal for school chemistry practicals and is often used to demonstrate supersaturation and nucleation.
Materials Required for Preparing Copper Sulphate Crystals
Before we dive into the method, gather the following items:
Required Materials:
- Copper sulphate powder (CuSO₄·5H₂O)
- Distilled water
- Heat-safe beaker or glass container
- Bunsen burner or heating stove
- Glass rod (for stirring)
- Filter paper and funnel
- Watch glass or saucer
- Safety gloves and goggles (for protection)
Note: Use lab-grade copper sulphate for best results.
Step-by-Step Procedure on how crystals of copper sulphate are prepared
Let’s break down the steps to describe how crystals of copper sulphate are prepared clearly and methodically.
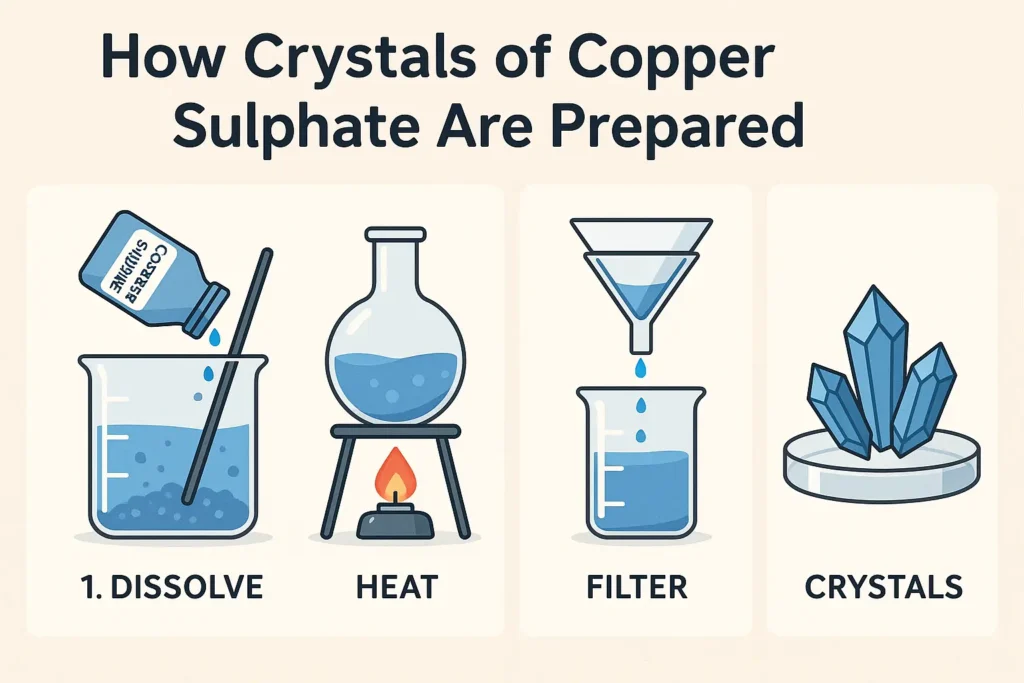
Step 1: Prepare a Saturated Solution
- Take 100 ml of distilled water in a beaker.
- Warm it gently over a burner.
- Add copper sulphate powder slowly while stirring until no more dissolves.
- This creates a hot saturated solution.
Tip: Do not boil the solution. Just warm it enough to dissolve more solute.
Step 2: Filter the Solution
- Once the solution is saturated, filter it using filter paper and a funnel.
- This step removes impurities and undissolved particles.
Step 3: Allow It to Cool
- Pour the clear solution into a clean glass container or saucer.
- Cover it with a watch glass or paper to prevent dust from entering.
- Let it cool undisturbed for 24–48 hours at room temperature.
This is when crystallization begins as the solution becomes supersaturated.
Step 4: Observe the Crystals
- After 1–2 days, you’ll see beautiful blue copper sulphate crystals forming at the bottom.
- Use tweezers to pick them up gently.
Step 5: Dry the Crystals
- Place the crystals on a filter paper or tissue to absorb moisture.
- Allow them to air dry completely.
Hope Now you this step by step process has properly describe how crystals of copper sulphate are prepared.
Congratulations! You’ve successfully grown copper sulphate crystals.
Queries Covered:
- Describe how crystals of copper sulphate are prepared
- how to grow copper sulphate crystals at home
- copper sulphate crystal preparation procedure
- chemistry lab experiment copper sulphate
- step by step copper sulphate crystal formation
- why do copper sulphate crystals form
Precautions to Take During the Experiment
Safety Tips:
- Always wear gloves and safety goggles.
- Do not ingest copper sulphate; it is toxic.
- Ensure the experiment is done in a well-ventilated area.
- Use distilled water only for best results.
- Do not disturb the solution once it’s cooling, or crystals may not form properly.
Scientific Principle Behind Crystallization
What is Crystallization?
Crystallization is a physical change where a solid forms from a solution due to supersaturation. As the hot solution cools, the solubility of copper sulphate decreases, causing it to come out of the solution and form solid crystals.
Key Concepts:
- Saturation: When no more solute can dissolve in the solvent.
- Supersaturation: A heated solution that holds more solute than normally possible.
- Nucleation: The initial step where particles come together to form a crystal.
Factors Affecting Crystal Formation
- Temperature: Higher temperatures dissolve more solute; cooling triggers crystallization.
- Purity of the solution: Impurities hinder crystal growth.
- Stillness: Movement disturbs crystal formation.
- Evaporation speed: Slow cooling = better, larger crystals.
Uses of Copper Sulphate Crystals
Copper sulphate crystals are more than just a lab experiment. They have practical uses, such as:
Common Uses:
- Agriculture: Used as a fungicide and pesticide.
- Education: Used in chemistry demonstrations and projects.
- Electroplating: Helps in copper plating processes.
- Medical: Historically used as an antiseptic.
- Algae Control: Used in ponds and water tanks.
FAQs: Describe How Crystals of Copper Sulphate Are Prepared
Can I grow copper sulphate crystals at home?
Yes, you can! Just ensure adult supervision and proper safety precautions if you’re trying it outside a lab.
How long does it take to form crystals?
It typically takes 24 to 48 hours for good-sized crystals to form under room temperature.
Why are my crystals not forming?
Possible reasons:
- Solution wasn’t saturated enough.
- Solution was disturbed during cooling.
- Impurities were present.
Are copper sulphate crystals safe to touch?
They are safe to touch with gloves, but never ingest them or touch your mouth/eyes afterward.
Can I reuse the leftover solution?
Yes, the remaining solution can be reheated to grow more crystals or reused in further experiments.
Key Takeaways
- The preparation of copper sulphate crystals demonstrates crystallization.
- It’s a simple experiment involving copper sulphate, water, heat, and patience.
- Understanding this helps build fundamental knowledge in physical chemistry.
- Proper safety measures are essential while handling chemicals.
Conclusion
To wrap it up, learning how to describe how crystals of copper sulphate are prepared is a must for any chemistry student or science enthusiast. It’s not only fun and visually satisfying but also educational. It teaches the science behind crystallization, the importance of laboratory procedures, and the practical application of theoretical knowledge.
With just a few materials and careful observation, you can grow beautiful, vibrant crystals and gain a deeper appreciation for chemical processes.