Introduction
In science and engineering, the concept of specific gravity is widely used to compare substances in a simple, dimensionless way. Instead of dealing with absolute density values (which require units), specific gravity expresses how heavy or light a substance is compared to a standard reference.
For solids and liquids, the reference is usually water at 4°C (because at this temperature water reaches its maximum density, 1000 kg/m³). For gases, the reference is air at standard conditions.
Specific gravity has been applied for centuries—from ancient methods of gold testing to modern applications in civil engineering, medical diagnostics, chemical industries, petroleum refining, food quality control, and environmental science.
Table of Contents
Definition of specific gravity
Specific Gravity (SG) is defined as the ratio of the density of a substance to the density of water (or air, for gases).
Standard Formula of specifc gravity
SG= Density of Current substance / Density of reference SG (Mostly water at 4 deg C)
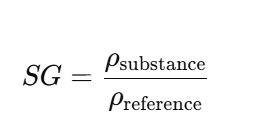
Key Characteristics:
- It is dimensionless (no units).
- It provides a quick comparison between materials.
SG > 1 → substance is heavier than water.
SG < 1 → substance is lighter than water. - SG helps identify substances and verify their purity
- Unlike density, SG is easier to compare across substances because it’s a pure number
- Indicator of Buoyancy
Determines whether an object will float or sink in water:
SG < 1 → floats
SG > 1 → sinks
what factors effect specific gravity value:
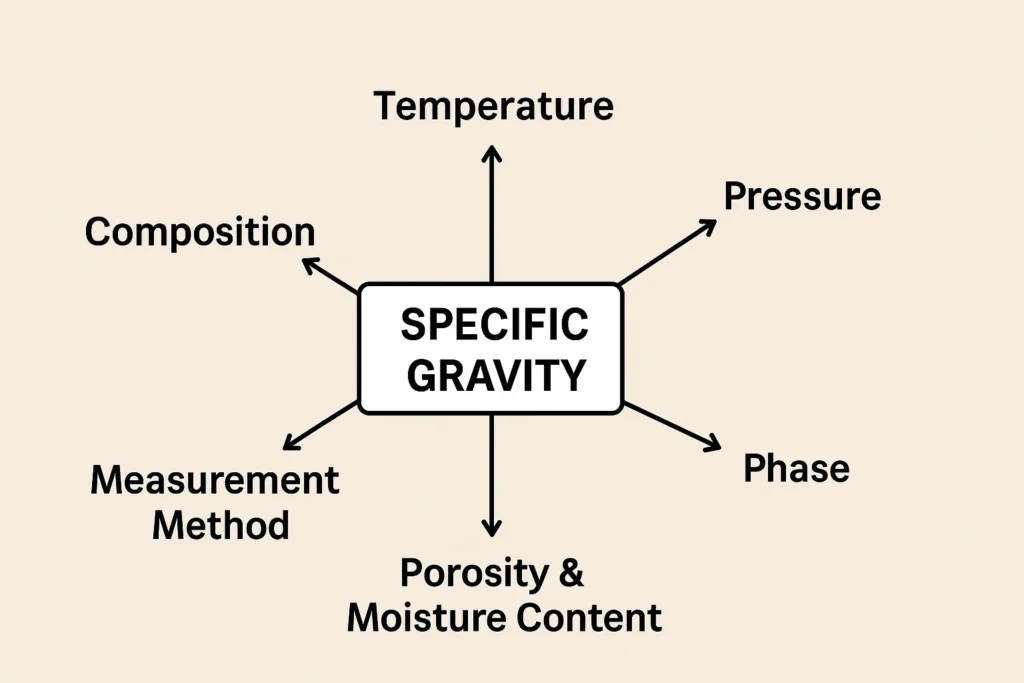
Temperature
As temperature increases, most substances expand, reducing their density.
Water, for example, has its maximum density at 4°C; at higher or lower temperatures, its density decreases.
This means the specific gravity of liquids and solids is temperature-dependent.
Example: Ethanol Specific gravity decreases when heated.
Pressure
Pressure has little effect on liquids and solids because they are nearly incompressible.
For gases, pressure has a significant effect: density increases with pressure, so specific gravity relative to air also changes
Purity and composition
Impurities, dissolved substances, or varying composition change the density, and therefore SG.
Example: Sea water (SG ≈ 1.025) vs. pure water (SG = 1.000).
Example: Urine Specific gravity increases with dissolved salts and glucose
Phase of substance
Solids, liquids, and gases of the same substance can have different densities.
Example: Ice (SG = 0.92) floats on liquid water (SG = 1.0).
Porosity and Moisture Content
In materials such as soil, sand, or cement, trapped air or absorbed water can alter the measured SG.
That’s why standard methods (pycnometer, Le Chatelier flask) are used to eliminate errors.
Measurement Method and Accuracy
Different instruments (hydrometer, pycnometer, digital density meter) have different levels of precision.
Errors in weighing or temperature control can affect the calculated SG.
What is the specific gravity of water:
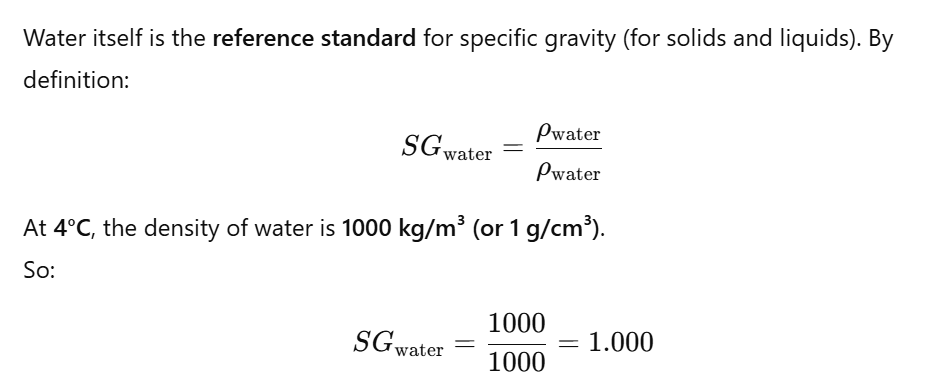
The specific gravity of pure water is always taken as 1.000 at 4°C.
At other temperatures, the density of water changes slightly:
- At 0°C → 0.9998 g/cm³
- At 20°C → 0.9982 g/cm³
- At 100°C → 0.958 g/cm³
But in practice, we still approximate SG of water = 1 (unless very high precision is needed).
List of Specific Gravitys of various substance:
The following table gives approximate values of specific gravity for common substances, useful as a quick reference.
NOTE: These are the standard values (Values may vary slightly depending on temperature, purity, and composition.)
| Substance | Specific Gravity (Approx.) |
|---|---|
| Water (4°C) | 1.000 |
| Urine (normal) | 1.005 – 1.030 |
| Blood | 1.050 – 1.060 |
| Cement | 3.15 |
| Soil (average) | 2.60 – 2.80 |
| Sand | 2.63 – 2.67 |
| Clay | 2.65 |
| Diesel | 0.82 – 0.85 |
| Petrol (Gasoline) | 0.72 – 0.78 |
| Crude Oil | 0.80 – 0.97 |
| Alcohol (Ethanol) | 0.789 |
| Milk | 1.028 – 1.035 |
| Mercury | 13.6 |
| Air (STP) | 0.0012 |
| Oxygen (relative to air) | 1.17 |
| Iron | 7.87 |
| Aluminium | 2.70 |
| Copper | 8.96 |
| Lead | 11.3 |
| Gold | 19.3 |
| Silver | 10.5 |
| Glass | 2.4 – 2.8 |
| Wood (varies) | 0.3 – 0.9 |
| Ice | 0.92 |
| Sea Water | 1.025 |
| Sulphuric Acid (98%) | 1.84 |
| Honey | 1.36 – 1.45 |
| Olive Oil | 0.91 – 0.93 |
| Vegetable Oils | 0.90 – 0.92 |
| Bottle (glass) | 2.4 – 2.6 |
| Battery (lead-acid, typical) | 1.2 – 1.3 |
| Coarse Aggregate | 2.5 – 2.9 |
| Soil (compact, varies) | 1.2 – 2.0 |
Methods of Calculating Specific gravity
- From Density
- Measure or look up density of the material.
- Divide by the density of water (1000 kg/m³ at 4°C).
- Example: Density of cement = 3150 kg/m³ → SG = 3.15.
- Archimedes’ Principle (Solids)
- Weigh the object in air (W₁).
- Weigh the object submerged in water (W₂).
- Formula:
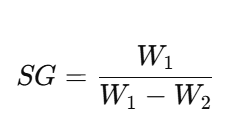
- Liquid Comparison
- Fill a container with equal volumes of liquid and water.
- Weigh both and take the ratio.
How to calculate specific gravity for different cases
Problem 1: Using Density
A liquid has a density of 850 kg/m³. Calculate its specific gravity.
SG=850/1000=0.85
Answer: SG = 0.85 (typical of diesel).
Problem 2: Solid Object
If a stone weighs 120 g in air and 90 g in water. Find its SG.
Archimedes Principal formula: (W1)/(W1-W2) = Specific gravity
W1 = 120 g
W2 = 90 g
SG= (120) / (120−90) = 4
Answer: Specifc gravity of solid object = 4
Problem 3: Medical Example
Urine sample density = 1.025 g/cm³. Find the specific gravity?.
SG=1.025 / 1.000=1.025
Answer: Specific gravity of urine = 1.025 (normal)
Problem 4: Cement
Cement density = 3150 kg/m³. Find the specific gravity?
SG= (3150) / (1000) = 3.15
Answer: Specific gravity of cement density is = 3.15
Problem 5: Gas Example
Density of oxygen = 1.429 kg/m³, density of air = 1.225 kg/m³.
SG= (1.429) / (1.225) = 1.17
Answer: Oxygen is 1.17 times heavier than air.
Methods of Measuring specific gravity
A. Laboratory / Instrument-Based Techniques
- Hydrometer: Floats in liquids, reading marked on the stem gives SG.
- Pycnometer (Specific Gravity Bottle): A small, calibrated glass bottle used for precise density measurement.
- Digital Density Meters: Automated and highly accurate instruments.
- Urinometer: Specialized hydrometer for urine SG in medicine.
B. Field / Simple Techniques
- Displacement Method: Volume of displaced water gives density → SG.
- Weight in Air and Water: Simple balance method.
- Household Approximation: Use kitchen scale and measuring cup.
Applications of Specific Gravity
- Civil Engineering
- Identifying quality of cement, soil, sand, and aggregates.
- Essential in mix design and soil classification.
- Medical Field
- Urine SG indicates hydration and kidney function.
- Blood plasma SG in diagnostic tests.
- Petroleum & Chemical Industries
- API gravity (in petroleum) derived from SG.
- Determines fuel quality and classification.
- Food & Beverage Industry
- Alcohol concentration in beer, wine, spirits.
- Checking purity of milk and honey.
- Environmental Science
- Understanding oil spills (floating vs. sinking oils).
- Studying groundwater and soil contamination.
- Everyday Uses
- Checking car battery acid (SG indicates charge).
- Explaining why ice floats in water.
FAQs of Specific gravity
Q1: What information does specific gravity provide?
Specific gravity indicates how heavy or light a substance is compared to water. It also helps predict whether an object will float or sink in the reference fluid.
Q2: How is specific gravity different from density?
Density is the mass per unit volume of a substance, while specific gravity is a dimensionless ratio of the density of a substance to the density of water.
Q3: Which metal is known for having a very high specific gravity?
Gold is one of the metals with the highest specific gravity, making it much heavier than most metals of the same size.
Q4: Which metal has the lowest specific gravity?
Lithium has the lowest specific gravity among common metals, making it extremely light.
Q5: How can we measure the specific gravity of liquids?
A hydrometer or a pycnometer is typically used to measure the specific gravity of liquids.
Q6: What does a blood specific gravity test indicate?
Blood specific gravity can provide information about hydration, blood concentration, and certain medical conditions when compared with normal ranges.
Q7: What does specific gravity mean for gemstones?
For gemstones, specific gravity helps identify the type of gem and distinguish it from imitations by comparing its density to water.
Q8: At what temperature does water reach maximum density?
Water has its maximum density at 4°C, which is used as a reference for specific gravity calculations.
Q9: What is the specific gravity of common rubber?
The specific gravity of natural rubber is approximately 0.92, making it lighter than water.
Q10: Why is specific gravity important in industry and science?
Specific gravity is widely used in geology, chemistry, and engineering to identify materials, assess purity, and determine buoyancy.
Q11: Can specific gravity change with temperature?
Yes, increasing temperature generally decreases the density of liquids and solids slightly, which can affect their specific gravity.
Q12: Can gases have specific gravity?
Yes, gases have specific gravity too. It’s calculated relative to air (for example, oxygen has a specific gravity of 1.11 relative to air).
Conclusion
Specific gravity is one of the most versatile and practical concepts in science and engineering. It eliminates the need for units, simplifies comparison, and has real-world applications ranging from construction quality control to medical diagnosis and fuel testing.
By mastering its calculation, measurement methods, and interpretation, students and professionals can quickly evaluate the physical properties of substances and apply this knowledge across industries.