Hey buddy, this blog is designed in a way that, you should understand the galvanic cell in a way that you could be able to visualise it and have clear ideas on what is galvanic cell, How does a galvaniccell works and be able to remember the diagram forever
Table of Contents
Before we begin understanding it, lets begin with the definition of galvanic cell
What is a Galvanic cell? Book definition:-
A galvanic cell (also known as a voltaic cell) is defined as:
“An electrochemical cell that converts chemical energy into electrical energy through a spontaneous redox reaction occurring in two separate half-cells.”
Now let’s understand in engineering bro way
What is a Galvanic cell, and how does it work?
Let’s begin with an example
🌟 The Tale of Two Brothers: Zinc and Copper
Once upon a time, in the land of Chemistry, there lived two brothers: Zinc and Copper. They were both metals, but with very different personalities.
Zinc was impulsive and energetic. He always wanted to give away things—even his precious electrons. On the other hand, Copper was calm and composed, not in a hurry to give, but he loved receiving—especially electrons.
One day, a wise chemist built a bridge between their homes (metal strips in different solutions) and called it a galvanic cell.
🧪 The Set-Up
The chemist placed:
- Zinc in a pool of zinc sulfate solution (ZnSO₄) and
- Copper in a pool of copper sulfate solution (CuSO₄).
These were their personal pools—each filled with their own metal ions.
He then connected them with:
- A wire — so electrons could run from Zinc to Copper.
- A salt bridge — a magical tunnel that kept the solutions balanced by letting ions flow without mixing the pools too much.
⚡ The Reaction Begins
Zinc, being the generous brother, gave away electrons and turned into Zn²⁺ ions, dissolving into his own solution.
This action is called oxidation.
The electrons traveled excitedly through the wire (this movement is what we call electric current) all the way to Copper.
Copper was waiting calmly and happily accepted the electrons, and Cu²⁺ ions from his solution got reduced—they turned into solid copper and stuck to him, making him grow stronger.
This is called reduction.
🌍 The Magic of the Galvanic Cell
- The spot where Zinc lost electrons is called the anode (remember: anode = oxidation).
- The spot where Copper gained electrons is the cathode (cathode = reduction).
- The salt bridge made sure the solution didn’t get too positive or too negative, by allowing the right ions to flow.
🎉 The Result
Thanks to this dance of electrons and ions, the chemist now had a working battery! This was a galvanic cell—a natural energy converter turning chemical energy into electrical energy.
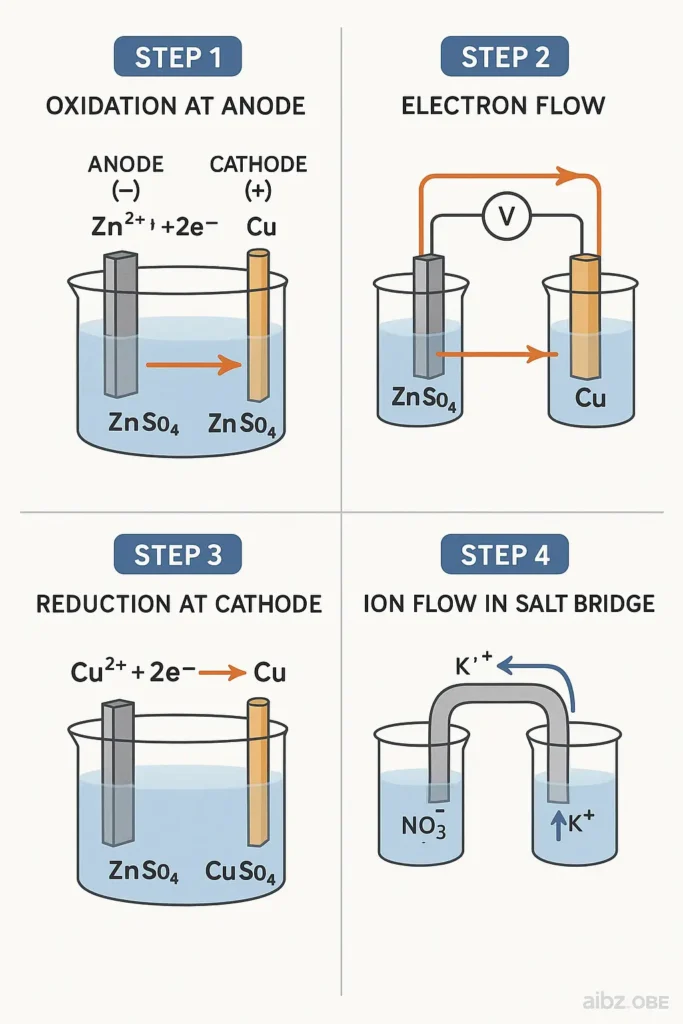
Key takeaways from this story
🔑 Key Concepts Simplified:
- Zinc is the Electron Donor (Anode)
- Zinc gives away electrons.
- It undergoes oxidation (Zn → Zn²⁺ + 2e⁻).
- It dissolves into its solution as ions.
- Copper is the Electron Acceptor (Cathode)
- Copper receives electrons.
- It undergoes reduction (Cu²⁺ + 2e⁻ → Cu).
- Copper ions from the solution stick to the copper metal.
- Electrons Flow Through a Wire
- From Zinc (Anode) to Copper (Cathode).
- This electron movement generates electricity.
- Salt Bridge Balances the Charges
- Prevents the solutions from becoming too charged.
- Lets ions flow to keep the cell electrically neutral.
- Chemical → Electrical Energy
- The entire cell converts the energy from a chemical reaction into usable electrical energy.
🧠 Bonus Memory Tips:
- Anode = Oxidation = Electron Loss
- Cathode = Reduction = Electron Gain
- Electrons always flow from Anode to Cathode.